ReDrugBC
Novel therapeutic approaches, based on drug repurposing, for high risk non muscle invasive bladder cancer driven by patients’ proteomic signatures

Figure 1. The CMap output when using as input the proteomic signature of patients with NMIBC. The presented compounds were top ranked candidates that can potentially reverse the proteomic signature of high-risk NMIBC (NPS1 subgroup) to low-risk NMIBC (NPS3 subgroup). Queries 1-13 represent the CMAP results when using as input different subsets of the patients’ proteomic signature. Reprinted from (2).
Drug identification based on the tissue molecular profile of patients with bladder cancer.
At first we aimed to identify existing de-risked drugs in silico that could be used for the treatment of high-risk NMIBC. To this end, we employed the next generation CMap (https://clue.io/) which is a commonly used signature-based computational approach for drug repurposing. CMap relies on the signature reversion principle, based on which drugs that have the potential to reverse the expression profile of a given set of hallmark genes for a particular disease have therapeutic potential for that disease. As input for the CMap analysis was used the proteomic signature from patients with NMIBC at low (NPS3) versus high-risk (NPS1), as defined from our previously published high-resolution tissue proteomics analyses (1). Among the retrieved compounds predicted to reverse this signature (CMAP output), six compounds were identified at highest relative scores (Figure 1) (2). These compounds belonged to mTOR inhibitors, tubulin inhibitors and caspase activators. As shown in Figure 1, the specific ATP-competitive inhibitor of mTOR, WYE-354, was ranked top (2).

Figure 2. The top ranked compounds that may reverse transcriptomics and proteomics features associated with aggressive bladder cancer belonged to PI3K and mTOR classes. Reprinted from (2).
Evaluation of the impact of WYE-354 in vitro on bladder cancer cell lines.
To elucidate the possible impact of the WYE-354 mTOR inhibitor onto the malignant phenotype of BC cells in vitro, a panel of multi-origin BC cell lines was employed. WYE-354 administration resulted in a significant reduction in the proliferation rate of all the tested BC cell lines in a concentration-dependent manner (2). WYE-354 also significantly reduced the growth of all the tested cell lines when the cells were grown in 3D cultures in matrigel (Figure 3) (2). The observed impact of WYE-354 on BC cell growth was executed via delaying proliferation without affecting cell viability (2).
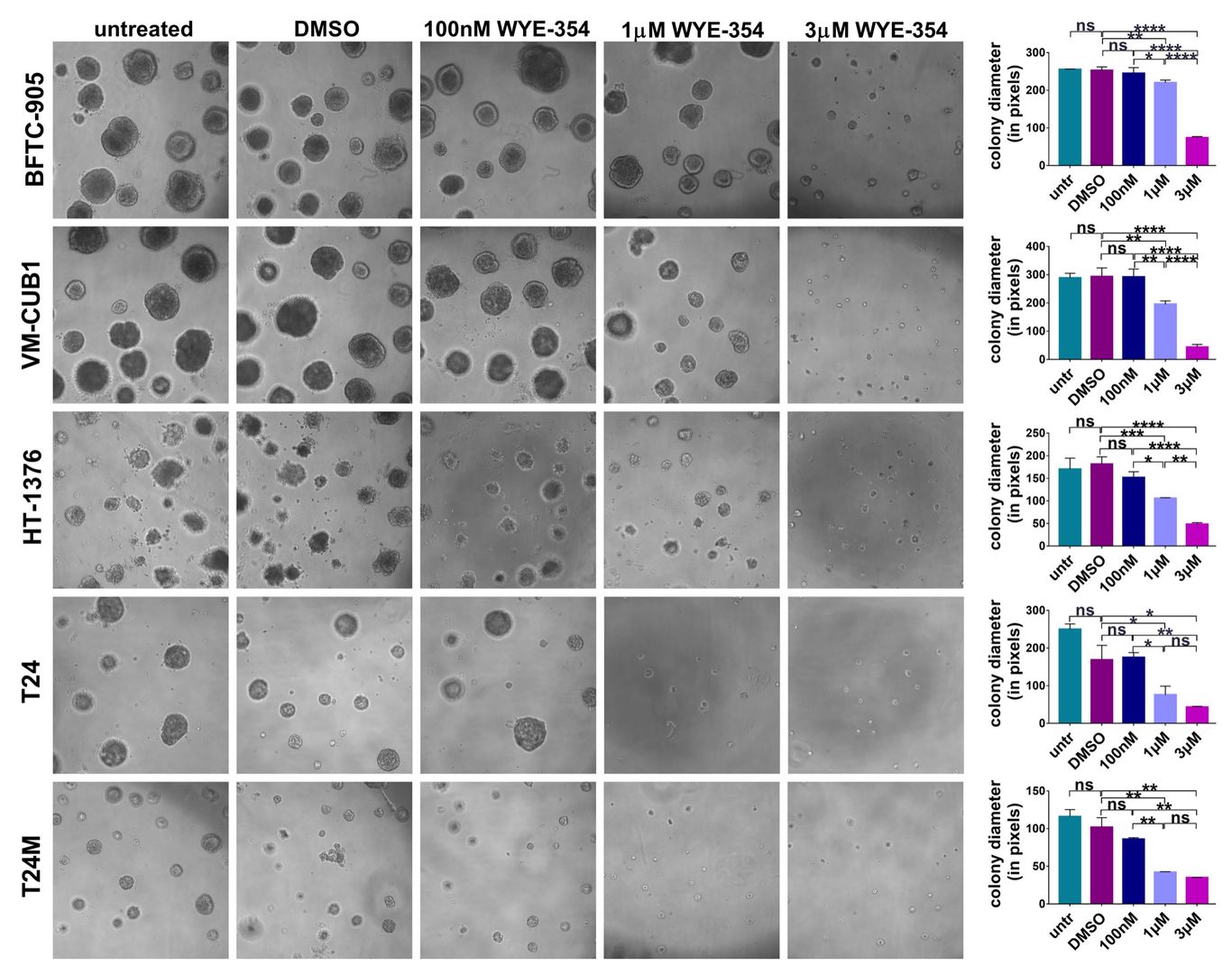
Figure 3. WYE-354 impact on BC cells growth in 3D cultures. A substantial decrease in the diameter of the colonies but not in their number was observed when the cells were treated with WYE-354 in comparison to those treated with DMSO or untreated cells. Reprinted from (2).

